Process of ferralitization
Table of contents
-
Introduction
-
Net loss of Silicium (Si) and formation of kaolinite
a. Example: hydrolysis of feldspar
-
Relative accumulation of sesquioxides
a. Oxidation of primary minerals
1. Introduction
|
 |
| Fig.1 Ferralic B horizon (here: Bo1 and
Bo2) |
| (
Source: www.soils.umn.edu/.../soil2125/ doc/s5chp2.htm ) |
|
-
Soils containing a ferralic B horizon could develop on
old continental platforms due to their geo-morphological stability over long
period of time and the exposure to wet and hot climate for extended periods of
time. Ferralitization is a slow process, also in the humid tropics.
|
-
Formerly this process was named laterization, kaolinization
or desilication.
-
The process comprises net loss of Si, formation of kaolinite
and relative accumulation of sesquioxides.
2. Net loss of Si and formation of kaolinite
-
Generally, hydrolysis reactions are most important processes
during chemical weathering. Water molecules split into their H and OH
components and the H often replaces a cation from the mineral
structure.
-
The net loss of Si and the formation of kaolinite during the
process of ferralitization will be explained by using the hydrolysis of
feldspars as an example.
-
Feldspars are highly abundant silicate minerals and are
stable in Earth‘s interior under high temperatures and little water. However,
they are very instable at Earth‘s surface (low temperature, abundant water).
 |
| Fig.2 Non-weathered compact granite: quartz (gray)
and feldspar (green) are tightly interlocked |
|
-
An extreme example of feldspar weathering is found in granite
blocks in the humid tropics due to heavy rainfalls, high temperatures and high
biological activity.
 |
| Fig.3 Grains of feldspar |
| (
Source: Press and Siever, 1995, p. 119.) |
a. Hydrolysis of feldspar
 |
| Fig.4 Feldspar in granite |
|
-
Water is used and is integrated into the lattice structure of
kaolinite. The H+ - ions of the water react with the
oxygen of the feldspar (water is adsorbed). The primary mineral disintegrates,
K and part of the Si are washed out in the soil solution resulting in the
formation of the secondary clay mineral kaolinite.
-
However, the reaction of feldspar with water under laboratory
conditions is a very slow process, i.e. this reaction cannot be responsible for
the rapid weathering in nature.
-
The addition of acid accelerates the process through the
release of H+-ions which may combine chemically with
other substances.
-
Carbon dioxide (CO2) is the most
abundant and natural occurring acid and responsible for the increase of the
weathering rate in nature.
-
It develops through solution of CO2 in
the rain water: H2O + CO2 to
H+ +
HCO3- ->
H2CO3
-
In rain water only very little CO2 is
dissolved because the concentration in the atmosphere is rather low (0,035 %).
However, it is sufficient for the weathering of feldspars. In comparison to
tropical soils, values up to 5 % were found through:
 |
| Fig.5 CO2 concentration (%, x-axis) in soil under
old-growth tropical rainforest (Costa Rica) |
| (
Source: Veldkamp, Göttingen.) |
-
The activity of microorganisms and plant roots which release
CO2 into soil air (acceleration of the process). Plants
and microorganisms are more active under warm and moist conditions and produce
more CO2.
-
Weathering rate increases with increasing temperature, i.e.
the chemical reactions take place faster with increasing temperature in the
humid tropics.
-
This high concentrations are also found in deeper layers,
leading to an acceleration of weathering of the parent materials. Under humid
tropical conditions, water percolates through the soil and takes dissolved
weathering products along. As a consequence no reaction equilibrium will be
reached and the rock disintegration continues.
 |
| Fig.6 Weathering reaction |
|
Relative accumulation of sesquioxides
 |
| Fig.7 Banded Fe formation, South Africa. |
| (
Source: www.iml.rwth-aachen.de/ Dmg/fe.html.) |
-
The formation of sesquioxides (Al/Fe oxides) from primary
minerals is a result of oxidation.
-
Oxidation is the chemical combination of an element with
oxygen.
-
It is an important process where rocks contain Fe and Mn
(2-divalent; for example pyroxene).
-
Fe exists in the primary mineral as divalent (ferrous form).
If exposed to air and during soil formation Fe becomes oxidized (looses an
electron) and becomes trivalent (ferric form).
-
In the air Fe reacts instantaneously with oxygen leading to
the release of electrons. Iron changes from Fe2+ to
Fe3+.
-
In the soil or water, the oxidation of
Fe2+ to Fe3+ takes place
in the lattice of the mineral. The silicate structure dissolves and
Fe2+ oxidizes to Fe3+.
The formed Fe2O3 is largely
insoluble.
-
This changes (valence electrons, ionic radius) leads to a
de-stabilization of the lattice structure of the mineral.
-
The increase of positive charges is partly counterbalanced by
the release of other cations (K, Mg...) out of the lattice, and the lattice
becomes instable.
-
Further disintegration follows and
H+ -ions hydrolytically disrupt Si-O-M bindings in
the lattice elsewhere.
-
Because many minerals contain
Fe2+, the weathering of a rock is mostly accompanied
by a brown or reddish coloration.
-
Fe-oxides and hydroxides are small particles. They often coat
bigger particles like quartz. When Fe-oxide particles are removed by weathering
the quartz grains become uncovered giving the soil the typical gray color of an
albic E horizon (see fig.8).
 |
| Fig.8 Albic E horizon |
| (
Source: http://soils.ag.uidaho.edu/soilorders/index.htm) |
a. Oxidation of primary
minerals
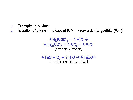 |
| Fig.9 Example olivine |
|
 |
| Fig.10 Example pyroxene |
|
-
For further pictures on primary minerals please click
 here here
|

 previous | next
previous | next

 previous | next
previous | next